Tests and Diagnosis of Liver Cancer
According to the PDQ Adult Treatment board, patients with large liver lesions more than 1cm in diameter who are also at risk for HCC should undergo testing involving imaging, biopsy or both in order to generate a proper diagnosis. Lesions smaller than 1cm in high-risk patients will be closely monitored, but will not undergo further testing for diagnosis since it is likely a cirrhotic lesion.
There are many negative predictors of survival from liver cancer. These include vascular invasion, or when the cancer cells escape into the blood vessels, fibrosis or the formation of excess connective tissue in an organ (in this case the liver) and cirrhosis. Tumor size is also a negative predictor.
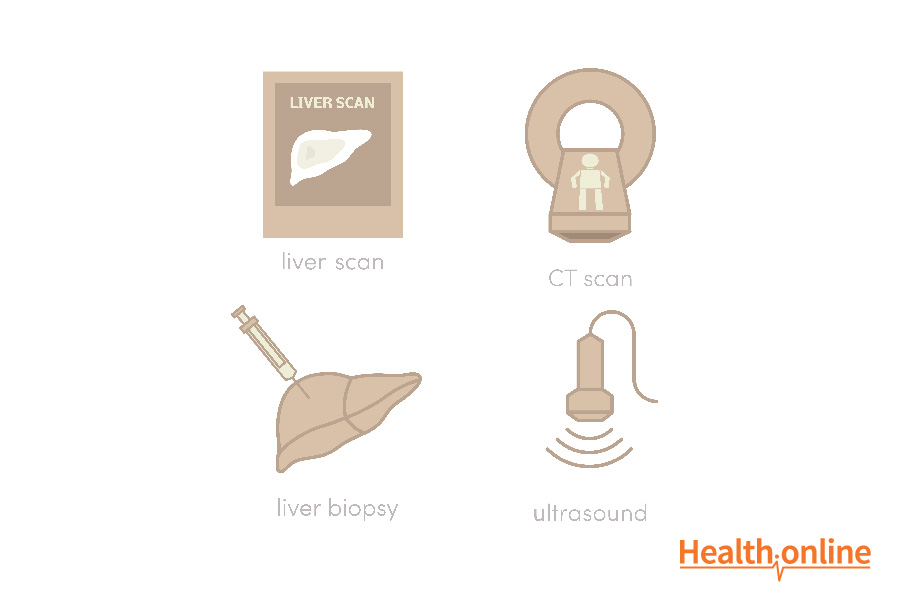
Imaging : Lesions are imaged using either triple-phase, contrast-enhanced computed tomography (CT) or magnetic resonance imaging (MRI). Using CT or MRI, the liver is imaged in two different phases; the arterial phase and the venous phase. Prior to imaging, a contrast media is injected into the patient’s veins to enhance the contrast of various structures in the different phases. Because HCCs only contain blood from the arteries while the rest of the liver has arteries and veins, the contrast media is more highly concentrated in the HCC. Images from the arterial phase display the HCC more prominently. However, in the venous phase, the HCC will be less prominent compared to the rest of the tumor since this phase emphasizes structures with veins. This imaging technique is highly specific and can be used to diagnose HCC without a biopsy.
Biopsy: If imaging fails to provide conclusive evidence of the liver lesion, a biopsy may be performed. The gold standard biopsy approach for the liver is known as the transjugular approach. The technique entails inserting a biopsy needle into the hepatic vein. Once the needle reaches the liver through the vein, a sample of liver cells is collected from the suspicious tissue.
In order to confirm a biopsy of HCC cells, the liver cells must show evidence of differentiation and malignant characteristics. Microscopically, the cells will appear to have large nuclei and nucleoli. Thus, there will be a high ratio of nucleus to cytoplasm. These cells will also likely display hyperchromatism or increased density of the DNA and other nuclear variations. The membranes of these cells are prominent and their cytoplasm appears fine and granular. Bile and cytoplasmic fat may be found in the tumor cells as well. Some tumor cells may contain cytoplasmic Mallory bodies or damaged intermediate filaments. HCCs grow in a way that is similar to normal liver cells. These tumors grow in a trabecular pattern.
CC cells are usually arranged in tubules and glands, although they may also be found in nests, cord and papillary structures. CCs are also highly differentiated with small nuclei that lack prominent nucleoli, unlike HCC cells. This cancer spreads throughout the liver through the vasculature.
Blood Tests: Serum may also be collected and tested for direct and indirect markers of liver disease. Indirect markers, or those that correlate with a disease state, but that is not involved with the development of the disease, include aspartate aminotransferase (AST), alanine aminotransferases, and the ratio of AST to platelets. Direct markers or byproducts of disease progression include hydaluronic acid, metalloproteinase-1, and procollagenase 3.
A less specific diagnostic assay that may be used is measurement of Alpha-fetoprotein (AFP) levels. AFP levels are typically elevated in cases of intrahepatic cholangiocarcinoma and metastases from colon cancer. While a diagnosis may not be made when high AFP levels are detected, the lesion may be monitored for progression.